Translate this page into:
Effects of Transmucosal Thyroxine Administration on the Tooth Movement in an Animal Model
Address for correspondence: Dr. Young-Guk Park, Department of Orthodontics, School of Dentistry, Kyung Hee University, 26, Kyungheedae-Ro, Dongdaemoon-Gu, Seoul, Korea. E-mail: ygpark@khu.ac.kr
This article was originally published by Wolters Kluwer and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
The aim of this study was to investigate the effects of transmucosal administration of thyroxine on the tooth movement and osteoclastic activity in Beagle dogs.
Materials and Methods
Eight Beagles were randomly divided into control group (n = 4) and thyroxine group (4-week group, n = 2 and 8-week group, n = 2). Buttons were bonded on the labial surfaces of the second premolar and anchorage teeth. Nickel-titanium closed-coil springs were connected. In the thyroxine group, thyroxine tablets were bonded to the hooks attached to the second premolar.
Results
The mean rate of orthodontic tooth movement (OTM) in the thyroxine group was slightly higher than that in the control group. Microscopic evaluation showed that the number of osteoclasts in the thyroxine group significantly increased.
Conclusion
The protocol for transmucosal administration of thyroxine could not significantly accelerate OTM. An increase in the number of osteoclasts was observed through microscopic evaluation during the 4th week.
Keywords
Accelerated orthodontic tooth movement
pharmacological treatment
thyroxine
Introduction
If there is an intervention that facilitates or inhibits the biological mechanism that is observed during orthodontic tooth movement (OTM), it will affect the tooth movement.[1]
Device-assisted therapy constitutes vast approaches to accelerate tooth movement. Recently, researches on resonance vibration, low-level laser, and photobiomodulation were utilized and positive results were reported.[2]
Surgical techniques also have been investigated in numerous studies. A variety of techniques, such as interseptal alveolar surgery, osteotomy, corticotomy, and Piezocision technique, have been used.[3] The two main features of regional acceleratory phenomenon in bone healing include decreased regional bone density and accelerated bone turnover, which are believed to facilitate OTM.[4,5]
In addition to these methods, pharmacological treatment may constitute another category of intervention. The relationship between the rate of OTM and systemic or local administration of medication has been explored on the basis that pharmaceutical agents may affect bone metabolism.[6,7]
Local, targeted administration of low doses of drug may limit the area of increased drug concentration, thus minimizing systemic effects. Administration of medicine in the form that has adhesive properties and attaches to the mucosa adjacent to the alveolar bone could promote OTM by increasing the local concentration while reducing systemic adverse effects.
The oral mucosa has many features useful in drug delivery. The keratinized layer in the mucosal surface and its permeability barrier are not as extensive as the skin stratum corneum. For easy diffusion of the pharmacological agent, lipid-soluble substances and nonionized species are required along with the characteristic of low molecular weights.[8,9]
In this study, we planned to use a method to apply a nondissolvable backing layer in order to augment the durability of mucoadhesive tablets, which can increase the exposure time and deliver the medication locally.
Exogenous thyroxine has several advantages. The size is small, with a molecular mass of 776.87 g/mol, and as a hormone, it can induce body reactions with a miniscule amount to the order of micrograms.
The thyroid hormone triiodothyronine (T3) and its prohormone thyroxine (T4), produced by the thyroid gland, are responsible primarily for regulation of metabolism. Thyroid hormones are involved in skeletal development in children and bone maintenance in adults.[10]
Adult bone structure and strength are maintained by a continuous process of bone turnover and repair through the bone remodeling cycle.[11]
The aim of this study was to investigate the effects of transmucosal administration of thyroxine on tooth movement and osteoclastic activity in Beagle dogs.
Materials and Methods
Since the Beagle dog was the target animal, the tablet form was selected for drug administration, and its dissolution was altered. A study by Ganesh et al. was referred to for the method of manufacturing a mucoadhesive polymer drug using natural polymer.[12] The process of tablet administration first involves dissolution at the attached location, followed by absorption into the oral mucosa [Figure 1]. Thus, the drug release profile and penetration across the epithelium of the oral mucosa are crucial in developing an effective delivery method.
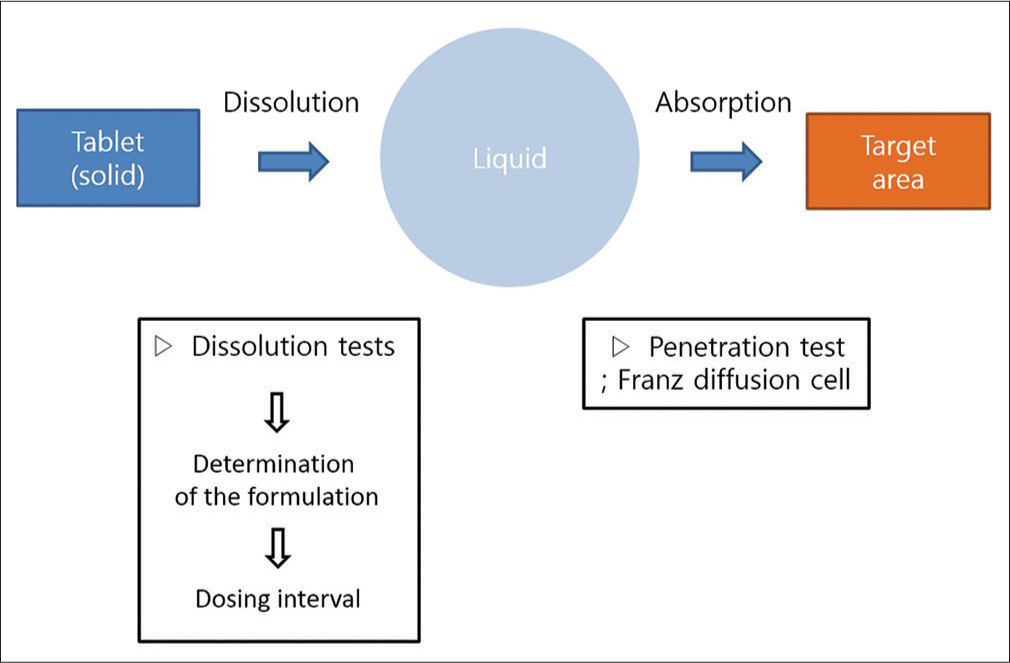
- The conceptual diagram of transmucosal drug delivery
Since the rate of dissolution determines the interval between doses, the rate of dissolution must be reduced while the rate of absorption into the mucosa is increased. Dissolution tests were therefore performed on various combinations of xanthan gum, sodium lauryl sulfate, fumed silica, mannitol, and magnesium-stearate to produce a formulation with these characteristics [Table 1]. A total of three formulations were manufactured with increasing xanthan gum/mannitol ratio, which determines the rate of dissolution (formulation numbers 1–3; F1–F3). The release ratio of each formulation was measured 2, 8, and 24 h after the start of dissolution. On the basis of the result from solubility tests, the formulation of F3 was selected.
| Formulation | F1 | F2 | F3 |
|---|---|---|---|
| Xanthan gum | 40.23 | 51.72 | 68.97 |
| Sodium lauryl sulfate | 1.72 | 1.72 | 1.72 |
| Fumed silica | 5.75 | 5.75 | 5.75 |
| Mannitol | 51.15 | 39.66 | 22.41 |
| Magnesium-stearate | 1.15 | 1.15 | 1.15 |
All values are given as percentages
Additional dissolution testing was conducted with the selected formulation for 1 week, the target dosing period. Specifically, four sample tablets were manufactured using the F3 formulation and detailed dissolution testing was conducted for 1 week. The release profile was observed 2 h after the start of dissolution, and then at 1-day intervals up to the 6th day.
Eight Beagles were used for the experiment. The inclusion criteria were male gender, 18–24 months of age, 10–13 kg body weight with all teeth present, and morphologically normal, with no systematic disease or symptoms.
They were randomly divided into two groups: the control group (C, n = 4) and the thyroxine administration group (T, n = 4). These groups were further divided into two subgroups based on the duration of force application: the 4-week groups (C-4 and T-4) and the 8-week groups (C-8 and T-8) [Figure 2].

- Experimental scheme. Blue circles indicate those in the thyroxine administration group, and yellow circles indicate the control group. During the period indicated by the orange box, distances of tooth movement were measured and compared
The target teeth were the second premolars of the upper and lower arches [Figure 3a]. The upper second premolars were protracted against the upper canines as anchorage. However, the lower second premolars were retracted against the fourth premolar and first molar because of anatomical limitations.

- Study design of orthodontic tooth movement and thyroxine tablet attachment. (a) Extraction site marked with arrows. (b) Target teeth with orthodontic appliances and thyroxine tablets
One week after extraction (T0), nickel-titanium closed-coil springs were connected to exert a force of approximately 100 cN per side. The force magnitude was measured using a force gauge and was reactivated on a weekly basis to exert a continuous force.
In the thyroxine administration groups, thyroxine tablets were bonded to the hooks attached to the target teeth using flowable composite [Figure 3b]. When placing the tablets, the hooks’ and tablets’ positions were adjusted to place the uncoated surface of the tablets in contact with the oral mucosa adjacent to the alveolar bone surrounding the roots of the target teeth [Figure 4]. Tablets were changed on a weekly basis.
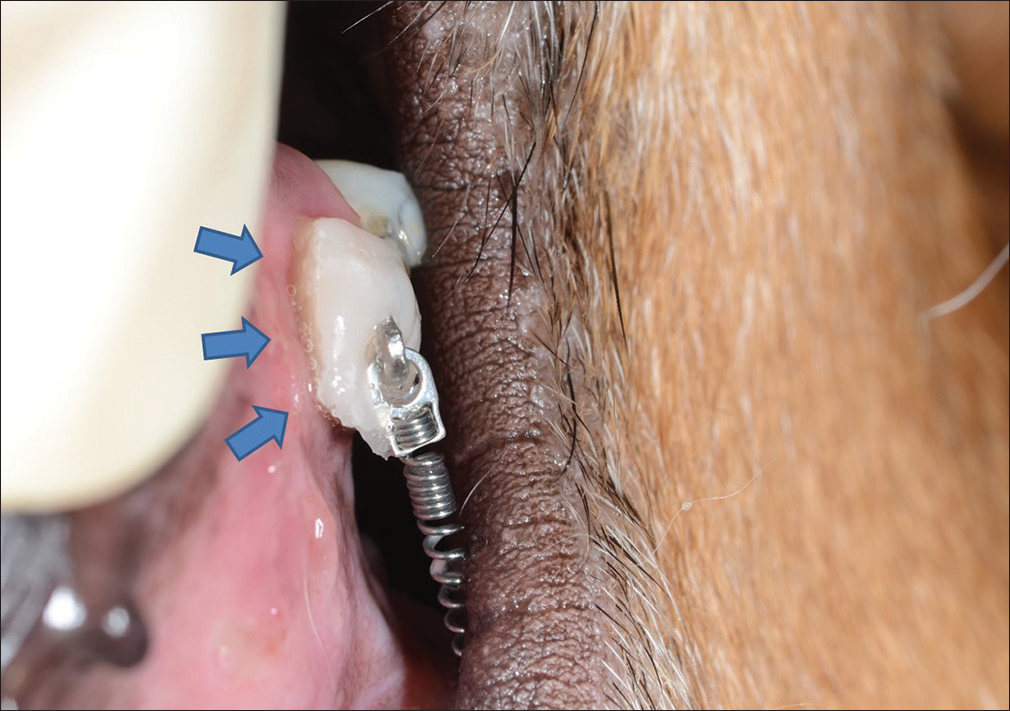
- Image of the thyroxine tablet dissolving inside the mouth. Even 3 days after adhesion, the drug is still securely attached. The dissolved drug (blue arrows) contacts the oral mucosa at the target site in the form of a viscous liquid
OTM was continued for 4 or 8 weeks, depending on the group. However, orthodontic force was applied to all the Beagles in the first 4 weeks; hence, the comparison of OTM was limited to this period, with weekly indirect measurements of OTM on stone models fabricated precisely using alginate impressions. A digital caliper was used to measure tooth movement on the fabricated models once a week. In the maxillary arch, the distance from the distal cervix of the third premolar to the mesial cervix of the moved second premolar was measured over time. In the mandibular arch, the distance from the mesial cervix of the canine to the mesial cervix of the moved second premolar was measured [Figure 5].

- Measurement of orthodontic tooth movement
At the end of OTM, tissue blocks including the second premolars with the surrounding alveolar bone were harvested. In addition, alveolar bone surrounding the upper canines was harvested. The purpose of harvesting the upper canine tissues was to evaluate systemic effects by comparing osteoclastic activities in areas other than the thyroxine target area. Sections were deparaffinized and stained for tartrate-resistant acid phosphatase (TRAP) using an acid phosphatase leukocyte according to the manufacturer’s recommended protocol, counterstained with eosin, and mounted for imaging and histomorphometry. This staining was performed to count the number of osteoclasts on the cervical third of the pressure-applied side [Figure 6].

- Tartrate-resistant acid phosphatase staining of a target tooth (×40). Tartrate-resistant acid phosphatase staining was performed to count the number of osteoclasts on the cervical third of the pressure-applied side (blue box)
To perform histomorphometry, images were obtained using an optical microscope connected to a computer, with a charge-coupled device camera. A slide that clearly showed the cervical third was randomly selected, and then three images in the relevant areas were randomly selected for counting. Cells were considered osteoclasts if they were multinucleated, TRAP positive. Counting was done manually under light microscopy.
Statistical analysis
Comparison of OTM between the groups – The normality of the distribution of the OTM distance data was assessed using the Shapiro–Wilk’s test. For normally distributed measurements, the independent t-tests were used to evaluate the intergroup differences of the tooth movement distances. For other measurements, the nonparametric Mann–Whitney U-tests were used. P < 0.05 was considered to indicate a statistically significant difference.
Comparison of osteoclastic activity – The number of osteoclasts was compared between thyroxine-applied group and control group. The normality of the distribution was assessed using the Shapiro–Wilk’s test, and the independent t-tests and the nonparametric Mann–Whitney U–tests were used where appropriate.
All tests were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA).
Results
Determination of the tablet formulations
The rate of dissolution was found to decrease with increasing xanthan gum/mannitol ratio [Figure 7]. The release ratios after 24 h for F1, F2, and F3 were 78.03%, 66.78%, and 18.69%, respectively. The probability to sustain the drug effect slowly and continuously for 1 week was the highest for F3. Among the formulations (F1–F3), F3 had the optimal rate of dissolution under the conditions of the experiment, with a formulation of 68.97% for xanthan gum, 1.72% for sodium lauryl sulfate, 5.75% for fumed silica, 22.41% for mannitol, and 1.15% for magnesium-stearate. Therefore, the formulation of F3 was selected.

- Dissolution test results and test conditions. The formulation of F3 was selected after considering 1 week of dosing interval
Comparison of orthodontic tooth movement between the groups
In the maxilla, the average distance of tooth movement was 0.17–0.20 mm/week in the control group and 0.16–0.21 mm/week in the thyroxine administration group. The average distance of tooth movement measured weekly was similar between the thyroxine administration and the control group. In the mandible, tooth movement was 0.15–0.19 mm/week in the control group and 0.18–0.20 mm/week in the thyroxine administration group on an average. The average distance of tooth movement in the mandible was also similar between the groups.
Table 2 and Figure 8 show accumulative distances of tooth movement by week. Both in the maxilla and the mandible, the pattern of tooth movement was very similar between the thyroxine administration and the control groups. In the mandible, the accumulative distances were somewhat greater in the thyroxine-treated group, but the difference was not statistically significant (P < 0.05).
| Jaw | Group | Mean±SD | |||
|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | ||
| Maxilla | Control (n=8) | 0.17±0.10 | 0.37±0.21 | 0.56±0.31 | 0.74±0.40 |
| Thyroxine (n=8) | 0.16±0.08 | 0.36±0.16 | 0.57±0.21 | 0.75±0.29 | |
| P | 0.958 | 0.713 | 0.563 | 0.955 | |
| Mandible | Control (n=8) | 0.15±0.07 | 0.34±0.15 | 0.50±0.20 | 0.70±0.25 |
| Thyroxine (n=8) | 0.19±0.08 | 0.37±0.13 | 0.57±0.18 | 0.76±0.25 | |
| P | 0.370 | 0.674 | 0.462 | 0.462 | |
All values are given in millimeter. P<0.05 was statistically significant. SD – Standard deviation

- Accumulative rate of orthodontic tooth movement. All values are given in millimeter. There was no significant differences between the control and thyroxine administration groups (P < 0.05)
The average rate of tooth movement by each week in the maxilla and the mandible was examined for the thyroxine administration and the control groups [Table 3]. First, the mean of the control group was 0.17–0.20 mm/week and 0.16–0.21 mm/week in the thyroxine administration group in the maxilla. At week 3, the rate shown by the thyroxine administration group was higher by 0.2 mm/week, indicating a faster tooth movement, but the overall rate was similar between the thyroxine administration and the control group. In the mandible, the mean rate of the control group was 0.15–0.19 mm/week and 0.18–0.20 mm/week in the thyroxine administration group. At weeks 1 and 3, in the thyroxine administration group, tooth movement was faster by 0.4 mm/week. P values were obtained using the Mann–Whitney U-tests when analyzing the week 1 maxilla data and the weeks 1 and 2 mandible data, as the normal distribution assumption was not met, and from independent t-tests when analyzing the data from other weeks.
| Jaw | Group | Mean±SD | ||||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Mean | ||
| Maxilla | Control (n=8) | 0.17±0.10 | 0.20±0.12 | 0.19±0.12 | 0.18±0.09 | 0.18±0.10 |
| Thyroxine (n=8) | 0.16±0.08 | 0.20±0.08 | 0.21±0.07 | 0.18±0.09 | 0.19±0.08 | |
| P | 0.958 | 0.960 | 0.645 | 0.956 | ||
| Mandible | Control (n=8) | 0.15±0.07 | 0.19±0.09 | 0.16±0.06 | 0.19±0.06 | 0.16±0.07 |
| Thyroxine (n=8) | 0.19±0.08 | 0.18±0.08 | 0.20±0.07 | 0.19±0.10 | 0.20±0.08 | |
| P | 0.370 | 0.792 | 0.222 | 0.875 | ||
All values are given in millimeter. P<0.05 was statistically significant. SD – Standard deviation
The mean rate of OTM for both upper and lower second premolars in the thyroxine group was slightly higher than that in the control; however, the difference was not statistically significant (P < 0.05). The transmucosal administration of thyroxine failed to accelerate tooth movement.
The fastest tooth movement over the 4 weeks was shown by C8-2 with the second premolar in the left maxilla moved by 1.38 mm. The same Beagle showed the fastest tooth movement in the mandible on the right, with a movement of 1.18 mm. In contrast, the slowest tooth movement in the maxilla over the 4 weeks was seen on the right side of T8-2 with a movement by 0.32 mm, and again the same Beagle showed the slowest tooth movement in the mandible, with the distance of tooth movement of 0.36 mm. The Beagle that showed the fastest tooth movement in the maxilla also showed fastest tooth movement in the mandible, and the Beagle that showed slowest tooth movement in the maxilla also showed the slowest tooth movement in the mandible. Large individual differences between Beagles were observed.
Microscopic evaluation of TRAP staining showed that the number of osteoclasts on the upper and lower second premolars in the T-4 group significantly increased by 1.50 folds for maxilla and 1.74 folds for mandible (P < 0.05). However, after 8 weeks of tooth movement, the number of osteoclasts in the 8-week groups (T-8 and C-8) was similar in intergroup comparison (0.84 fold for maxilla and 1.06 folds for mandible). The number of osteoclasts in the 8-week groups was lower when compared with that of the 4-week groups [Table 4 and Figure 9]. The number of osteoclasts in the upper canines was similar among the groups with the same period of tooth movement [Table 5].
| Jaw | Group | Mean±SD | P |
|---|---|---|---|
| Maxilla | C-4 group (n=4) | 10.50±1.29 | 0.002* |
| T-4 group (n=4) | 15.75±1.50 | ||
| C-8 group (n=4) | 4.75±2.50 | 0.620 | |
| T-8 group (n=4) | 4.00±1.41 | ||
| Mandible | C-4 group (n=4) | 8.75±0.96 | 0.003* |
| T-4 group (n=4) | 15.25±2.50 | ||
| C-8 group (n=4) | 4.50±1.29 | 0.823 | |
| T-8 group (n=4) | 4.75±1.71 |
*P<0.05 was statistically significant. SD – Standard deviation

- (a) Histologic view of C4-1 and T4-1 by tartrate-resistant acid phosphatase staining. All Teeth are lower left second premolars. The red arrows indicate osteoclasts. P and T indicate each pressure-applied side and tension-applied side. Red arrows indicate osteoclasts. (b) Histologic view of C4-2 and T4-2 by tartrate-resistant acid phosphatase staining. The number of osteoclasts in the T-4 group increased. (c) Histologic view of C8-1 and T8-1 by tartrate-resistant acid phosphatase staining. (d) Histologic view of C8-2 and T8-2 by tartrate-resistant acid phosphatase staining. The number of osteoclasts was similar in intergroup comparison
| Group | Mean±SD | P |
|---|---|---|
| C-4 group (n=4) | 5.00±2.16 | 0.750 |
| T-4 group (n=4) | 4.50±2.08 | |
| C-8 group (n=4) | 4.50±2.52 | 0.883 |
| T-8 group (n=4) | 4.75±2.06 |
P<0.05 was statistically significant. SD – Standard deviation
Discussion
In this study, the force used in tooth movement was 100 cN. In the study of Von Böhl et al.[13] using different magnitudes of continuous force on Beagles, it was reported that the amount of force used and the distance of OTM are not related, and individual differences have a greater impact on it. Studies by Kim et al.[14] and Ahn et al.[15] were referred to for the design of orthodontic force system in this study. Although the magnitude of force was adjusted using a force gauge, difference in the degree of force attenuation may arise over time and as the amount of spring activation changes.
No significant difference in the rates of tooth movement were observed between the thyroxine-treated and control groups. Therefore, the localized thyroxine application protocol used in this study was incapable of speeding up the tooth movement.
There were several limitations in the study. Those are small sample size, absence of quantitative evaluation of local drug delivery, and inconsistent drug delivery rate of manufactured tablets.
The small sample size is the biggest limitation of this study. In this study, the left and the right sides of each Beagle were treated as individual site. When an animal study is conducted using such an approach, there is a risk that experimental results turn insignificant if the animal variance is large. However, we decided that assigning each Beagle to either the thyroxine administration group or the control group would better fit the objectives of this study, rather than designing the experimental and control conditions for each Beagle, as there is a possibility for drug effects to present at systemic delivery even at low doses of the drug.
The major advantage of split-mouth design is its efficiency in terms of sample size. Split-mouth design was not used in this study since there is a possibility for drug effects to present at systemic delivery even at low doses of the drug. If there is a method to assure that administration of thyroxine on one site does not carry across to other quadrants, split-mouth design can be applied. Otherwise, the only way is to increase the sample size enough to offset the individual differences.
Conclusion
The rate of OTM in the thyroxine group was found to be slightly higher than that in the control group; however, the difference was not statistically significant (P < 0.05). The number of osteoclasts in the region of the upper and lower second premolars in the T-4 groups significantly increased by 1.50 folds for maxilla and 1.74 folds for mandible. However, the number of osteoclasts in the 8-week groups was similar in intergroup comparison. The following points can be considered:
The protocol for transmucosal administration of thyroxine used in this study could not accelerate OTM
An increase in the number of osteoclasts was observed through microscopic evaluation during the 4th week.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 1995;108:380-8.
- [Google Scholar]
- Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2008;133:572-83.
- [CrossRef] [PubMed] [Google Scholar]
- In vivo bioelectric petentials in the dentoalveolar complex. Am J Orthod. 1974;66:130-9.
- [Google Scholar]
- Corticotomy-/osteotomy-assisted tooth movement microCTs differ. J Dent Res. 2008;87:861-7.
- [CrossRef] [PubMed] [Google Scholar]
- Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: Histology and immunostaining. Am J Orthod Dentofacial Orthop. 2009;136(770):e1-11.
- [CrossRef] [PubMed] [Google Scholar]
- Influence of inflammation-mediated osteopenia on the regional acceleratory phenomenon and the systemic acceleratory phenomenon during healing of a bone defect in the rat. Calcif Tissue Int. 1998;63:160-6.
- [Google Scholar]
- Medication effects on the rate of orthodontic tooth movement: A systematic literature review. Am J Orthod Dentofacial Orthop. 2009;135:16-26.
- [Google Scholar]
- The effect of drugs on orthodontic tooth movement. Orthod Craniofac Res. 2006;9:163-71.
- [Google Scholar]
- Diffusion rates and transport pathways of fluorescein isothiocyanate (FITC)-labeled model compounds through buccal epithelium. Pharm Res. 1994;11:83-9.
- [Google Scholar]
- Thyroid hormone metabolism in skeletal development and adult bone maintenance. Trends Endocrinol Metab. 2012;23:155-62.
- [Google Scholar]
- Mechanisms of action of thyroid hormones in the skeleton. Biochim Biophys Acta. 2013;1830:3979-86.
- [Google Scholar]
- Design and development of buccal drug delivery system for labetalol using natural polymer. Int J Pharm Res Dev. 2011;3:37-49.
- [CrossRef] [Google Scholar]
- Changes in the periodontal ligament after experimental tooth movement using high and low continuous forces in Beagle dogs. Angle Orthod. 2004;74:16-25.
- [CrossRef] [Google Scholar]
- Effect of piezopuncture on tooth movement and bone remodeling in dogs. Am J Orthod Dentofacial Orthop. 2013;144:23-31.
- [Google Scholar]
- Timing of force application affects the rate of tooth movement into surgical alveolar defects with grafts in beagles. Am J Orthod Dentofacial Orthop. 2014;145:486-95.
- [Google Scholar]






